仪睿生物携手齐齐哈尔医学院,共绘生物医药创新协同新篇章!
发布时间:2024-08-30 10:47:00Article
Design, Synthesis, and Anticancer Activity of Novel Enmein-Type Diterpenoid Derivatives Targeting the PI3K/Akt/mTOR Signaling Pathway
Jiafeng Wang 1,† , Lu Wang 2,† , Yingbo Zhang 1 , Siwen Pan 1 , Yu Lin 2 , Jiale Wu 3 and Ming Bu 2, *
1 College of Pathology, Qiqihar Medical University, Qiqihar 161006, China; wangjiafeng410323@163.com (J.W.); zhangyingbo0930@163.com (Y.Z.); siwen_tongtong0606@163.com (S.P.)
2 College of Pharmacy, Qiqihar Medical University, Qiqihar 161006, China; wanglu9264582022@163.com (L.W.); linyu7373@163.com (Y.L.)
3 College of Life and Health, Hainan University, Haikou 570228, China; wwwwjl55@163.com
* Correspondence: buming@qmu.edu.cn; Tel.: +86-0452-2663-881
† These authors contributed equally to this work.
Abstract: The enmein-type diterpenoids are a class of anticancer ent-Kaurane diterpnoids that have received much attention in recent years. Herein, a novel 1,14-epoxy enmein-type diterpenoid 4, was reported in this project for the ffrst time. A series of novel enmein-type diterpenoid derivatives were also synthesized and tested for anticancer activities. Among all the derivatives, compound 7h exhibited the most signiffcant inhibitory effect against A549 cells (IC50 = 2.16 µM), being 11.03-folds better than its parental compound 4. Additionally, 7h exhibited relatively weak anti-proliferative activity (IC50 > 100 µM) against human normal L-02 cells, suggesting that it had excellent antiproliferative selectivity for cancer cells. Mechanism studies suggested that 7h induced G0/G1 arrest and apoptosis in A549 cells by inhibiting the PI3K/AKT/mTOR pathway. This process was associated with elevated intracellular ROS levels and collapsed MMP. In summary, these data identiffed 7h as a promising lead compound that warrants further investigation of its anticancer properties.
Keywords: enmein-type ent-Kauranes; derivatives; structural modiffcation; anticancer; PI3K/Akt/ mTOR pathway
Introduction
Cancer, the disease of abnormal cell proliferation, stands as one of the principal threats to human life in the 21st century [1]. According to recent statistics, the global cancer-related death toll reached 10 million in 2020, with lung cancer claiming the highest number of lives, accounting for 18.0% of total cancer deaths, followed by liver cancer (8.3%) and stomach cancer (7.7%) [2]. The global cancer mortality rate is increasing annually, leading to an estimated 16 million future deaths per year by 2040 [3]. With advancements in science and technology, state-of-the-art diagnostic techniques and therapeutic methods have been applied to control various types of cancers, improving the survival of patients to some extent [4–7]. Chemotherapy is one of the broadest cancer treatment strategies in clinical application, but the conventional chemotherapeutic agents are often prone to developing cancer cell resistance or causing serious poisonous side effects to patients [8]. This urgent health crisis is propelling the need for innovative therapeutic agents with higher targeting efffciency and minimal side effects [9,10]
Reagents and Apparatus
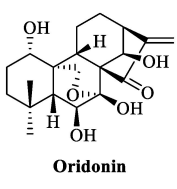
The starting material, Oridonin, was purchased from Chengdu Yirui Biotechnology Co., Ltd. (Chengdu, China), and the other reagents and solvents were purchased from Anegi (Shanghai, China) Medicinal Chemicals Co. All reagents were used directly after purchase and required no extra preparation. The chemical reactions were monitored using thin-layer chromatography, and the crude target products were puriffed using silica gel column chromatography. The silica gel preforms (model: HSGF254) and silica gel (100–200 mesh) were purchased from Yantai Yinlong Silica Gel Co (Yantai, China). The HRMS data were recorded on a Waters XEVO G2-XS QTOF high-resolution mass spectrometer. The NMR spectra were recorded on a Bruker AVANCE NEO 600 spectrometer, Fällanden, Switzerland, with CDCl3 or DMSO as solvent and tetramethylsilane (TMS) as internal standard. Coupling constants and chemical shift values are expressed in J (Hz) and d (ppm), respectively. The structures of all reported derivatives were consistent with the data from HRMS, 1H NMR, and 13C NMR spectra. The spectra of all derivatives are contained in Supplementary Materials.
文章链接:https://doi.org/10.3390/molecules29174066